The processing of temporal pitch and melody information in auditory cortex
From CNBH Acoustic Scale Wiki
An fMRI experiment was performed (with sparse imaging) to identify the main stages of melody processing in the auditory pathway. Spectrally matched sounds that produce no pitch, fixed pitch, or melody were all found to activate a large region in Heschl’s gyrus (HG) and planum temporale (PT). Within this region, sounds with pitch produced more activation than those without pitch in the lateral half of HG. When the pitch was varied to produce a melody, there was more activation than for fixed-pitch sounds, but only in regions beyond HG and PT, specifically in the superior temporal gyrus (STG) and lateral part of planum polare (PP). The results support the view that there is hierarchy of pitch processing and that the center of activity moves anterolaterally away from primary auditory cortex as the processing of melodic sounds proceeds, becoming asymmetric beyond HG with more activation in the right hemisphere.
Roy Patterson , Stefan Uppenkamp, Ingrid Johnsrude, Timothy Griffiths
Contents |
INTRODUCTION
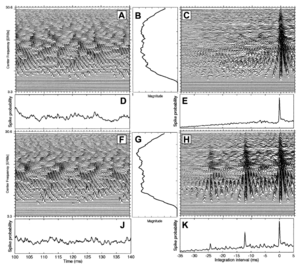
This paper is concerned with three auditory processes involved in the perception of melody, and how these processes are organized in the ascending auditory pathway. A melody in this case is simply a sequence of notes like that produced when someone picks out a tune on the piano with one finger. From the auditory perspective, perception of a melody involves (i) detecting that segments of an extended sound that contain temporal regularity, (ii) determining the pitch of each of these regular segments, and (iii) determining how the pitch changes from note to note over the course of the sound. Physiological studies (Palmer and Winter, 1992) and functional neuroimaging (Hall et al., 2002, Griffiths et al., 2001, Wessinger et al, 2001) suggest that the processing of temporal regularity begins in the brainstem and that pitch extraction is completed in HG, the site of primary auditory cortex (PAC) (Rademacher et al., 1993, 2001; Morosan et al., 2001; Rivier and Clarke, 1997; Hackett et al., 2001). This is consistent with the hierarchy of processing proposed for auditory cortex on the basis of recent anatomical studies in the macaque (Hackett et al., 1998, 2001; Kaas and Hackett, 2000; Rauschecker and Tian, 2000). Higher-level processes like pitch tracking and melody extraction are thought to be performed in more distributed regions beyond PAC, and the processing becomes asymmetric with more activity in the right hemisphere (see Zatorre et al., 2002 for a review). The current paper presents cortical data from a functional magnetic-resonance-imaging (fMRI) study designed to increase the sensitivity of auditory imaging and enable us to locate neural centers involved in pitch and melody perception with much greater precision. Studies of pitch processing often employ sinusoids that activate focal regions on the basilar membrane; these studies show that the ‘tonotopic’ organization observed in the cochlea is preserved in all of the nuclei of the auditory pathway up to PAC (for a review see Ehret and Romand, 1997). It is also possible to produce a tone with a strong pitch by regularizing the time intervals in a broadband noise, so that one time interval occurs more often than any of the others (see Figure 1). As the degree of regularity increases, the hiss of the noise dies away and the pitch of the tonal component increases to the point where it dominates the perception. These regular-interval (RI) sounds (Yost, 1998) are like noise insofar as they produce essentially uniform excitation along the basilar membrane, and thus, uniform activity across the tonotopic dimension of neural activity in the auditory pathway (compare panels B and G of Figure1). The fact that they produce a strong pitch, without producing a set of harmonically related peaks in the internal spectrum, shows that pitch can be coded temporally as well as tonotopically in the auditory system. Figure 1 illustrates how the auditory system could extract the pitch information from RI sounds. A brief comparison of spectral and temporal models of pitch is presented in Griffiths et al. (1998). RI sounds are useful in imaging because they enable us to generate sets of spectrally matched stimuli that enhance the sensitivity of perceptual contrasts in functional imaging. Their value was initially demonstrated by Griffiths et al. (1998) who used positron emission tomography (PET) to show that activation in HG increases with the temporal regularity of RI sounds and that, when the pitch changes over time, there is additional activation in STG and PP. The power was limited, however, by constraints on radiation dose, and spatial resolution was poor, compared to that of fMRI. For these reasons, the results were restricted to group data and they are ambiguous with regard to the degree of asymmetry at different stages. Subsequently, Griffiths et al. (2001) showed that the combination of RI sounds and fMRI was sufficiently sensitive to image all of the subcortical nuclei of the auditory pathway simultaneously, provided the technique included cardiac gating (Guimaraes et al., 1998) and many replications of each stimulus condition. A contrast between the activation produced by RI sounds with fixed pitch and spectrally matched noise revealed that temporal pitch processing begins in subcortical structures. At the same time, a contrast between sounds with varying pitch and fixed pitch did not reveal an increase in activation in this region. The fact that pitch processing begins in the brainstem but is not completed there was interpreted as further evidence for the hypothesis that there is a neural hierarchy of melody processing in the auditory pathway (Griffiths et al., 1998). In this paper, we present the cortical data from the fMRI experiment. The exceptional sensitivity of the study enables us to track the hierarchy of melody processing in auditory cortex across HG and out into PP and STG, and determine where the asymmetries reported by Zatorre et al. (2002) first emerge. Studies of cytoarchitecture have shown that a reliable landmark for primary auditory cortex is the anteriormost, transverse temporal gyrus (of Heschl) (Rademacher et al., 1993, 2001; Rivier and Clarke, 1997; Morosan et al., 2001), and functional imaging studies have shown that most complex sounds produce activation in PAC and surrounding areas in all normal listeners. The sensitivity of the cortical data means that we can investigate whether there are consistent differences between individual listeners in the location of functional activation within auditory cortex, and whether the differences correspond to differences in the sulcal and gyral morphology of the individuals (Penhune et al., 1996; Leonard et al., 1998).
RESULTS
The anteriormost transverse temporal gyrus of Heschl was identified in each of our listeners, and there was good agreement between this specification of the location of HG in our listeners and that obtained in other studies. The details of the analysis are presented in Experimental Procedures. The group activation results are presented first with respect to the average position of HG for the group. Then the variability of the activation across listeners is compared to the variability of HG across listeners.
Regions of Activation in the Group
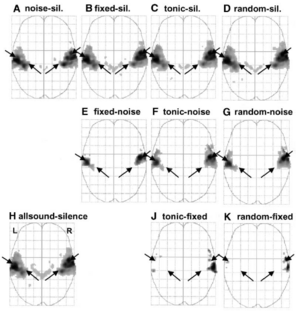
All sounds vs silence: The activation produced by all four sound conditions was compared to activation produced in the silence condition to illustrate the domain of cortical sensitivity to sound; the contrast includes 2592 volumes from all nine listeners (fixed-effects analysis). In cortex, this contrast yields bilateral activation in two large clusters shown in panel H of Figure 2 (allsound-silence). The clusters are centred in the region of HG and PT, as would be expected. Outside this region, there is essentially no other cortical activation, perhaps because it was a passive listening experiment. The ‘V’ of activity in the centre of the panel is the subcortical activity reported in Griffiths et al. (2001). The position of HG for the group of listeners falls along the line between the arrowheads in each hemisphere. The group activation is centred on the mean position of HG in the right hemisphere and along the posterolateral side of HG in the left hemisphere.
Individual sound conditions vs silence: The individual sound conditions produced very similar patterns of activation when compared to silence, as shown in panels A-D of Figure 2. In the left hemisphere, there is little to distinguish among the four contrasts in terms of the region of activation; in the right hemisphere, the three sounds with pitch produce slightly more activity in the region just anterior to the lateral end of HG. Many of the peaks in these contrasts have t-values above 10, ranging in some cases up to 40, and these peaks appear with remarkable consistency in all of the contrasts involving sound and silence. This means that the major peaks in the allsound vs silence condition are not the result of averaging peaks in slightly different positions in the separate conditions; these peaks occur in exactly the same place in most of the separate sound vs silence conditions. There are significant differences between conditions but they are largely associated with different levels of activation at fixed positions within the main clusters.
Differential sensitivity to pitch:
Differential sensitivity to melody: To reveal regions associated with melody processing, we examined the contrasts diatonic vs fixed and random vs fixed (panels J and K of Figure 2). Both contrasts reveal differential activation to melody in STG and PT, but the regions are relatively small and the activation is asymmetric with relatively more activity in the right hemisphere. In lateral HG and medial HG, there is virtually no differential activation when compared with the previous contrasts, indicating that melody produced about the same level of activity as fixed pitch in HG. This suggests that HG is involved in short-term, rather than longer-term, pitch processing, such as determining the pitch value or pitch strength, rather than evaluating pitch changes across a sequence of notes.
Diatonic vs random melody: In an attempt to identify regions that might be specifically involved in processing diatonic melodies, we examined the contrasts diatonic vs random and random vs diatonic. Neither contrast revealed significant peaks in any region of the brain. As a result, the data from the two melody conditions will be considered together in most of the following discussions.
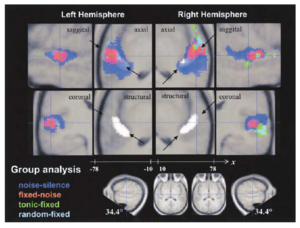
Hierarchy of melody processing: A summary of the results to this point is presented in Figure 3: the structural and axial sections show the activity in a plane parallel to the surface of the temporal lobe and just below it; the sagittal sections are orthogonal to the axial sections and they face outwards so that in both cases, the view of the temporal lobe is from outside the head. The highlighted regions in the structural sections show the average position of HG in the two hemispheres; they are replotted under the functional activation in the axial sections above. The functional activation shows that, as a sequence of noise bursts acquires the properties of melody (first pitch and then changing pitch), the region sensitive to the added complexity changes from a large area on HG and PT (blue), to a relatively focused area in the lateral half of HG (red), and then on out into surrounding regions of PP and STG (green and cyan mixed). The orderly progression is consistent with the hypothesis that the hierarchy of melody processing that begins in the brainstem continues in auditory cortex and subsequent regions of the temporal lobe. The activation is largely symmetric in auditory cortex and becomes asymmetric abruptly as it moves on to PP and STG with relatively more activity in the right hemisphere.
Variability in Anatomy and Functional Activation across Listeners
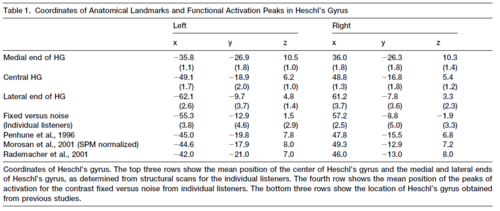
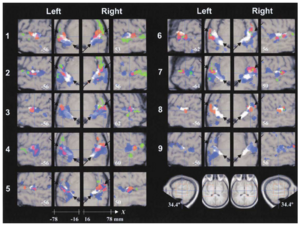
In this section, we examine how the anatomy of Heschl’s gyrus in individuals relates to the individual’s pattern of functional activation, and how the functional data of individuals relates to the pattern of activation observed in the group data. The analysis of the anatomy of HG was summarized for each listener in terms of three points: (i) the centroid of the complete volume of HG, (ii) the position of the medial end of HG, and (iii) the position of the lateral end of HG (see Experimental Procedures). The group centroids for each of these points are presented in Table 1 with the standard deviations; the table shows that, following normalisation, the variability in the position of HG is minimal. Specifically, the standard deviations for the medial and central centroids are less than one voxel (2 mm) in all three dimensions, and the standard deviations for the lateral centroid are less than two voxels, on average. With regard to the functional activation, the positions of the major peaks are very consistent across conditions within individuals. Nevertheless, the major peaks in the group data do not coincide with those in the data of individuals. Indeed, there are essentially no peaks in the group data that appear consistently for individuals. So, the position of a peak in the group data represents a location where activation from individuals overlaps in some way; it is not the location where a majority of the individuals all exhibit the same peak. In order to understand the form of the variability across listeners, axial and sagittal sections like those in Figure 3 were prepared for all nine listeners. The sections are presented with one listener per row in Figure 4. The first thing that the figure reveals is that the regions of activation in individuals are more focal than in the group average, indicating that the larger regions of activation in the group data represent collections of focal regions from individuals – regions which in an individual are highly consistent across conditions. Moreover, the variability in activation is different in the three contrasts: noise vs silence (blue), fixed vs noise (red) and melody vs fixed (green). The variability is analysed in the remainder of this section separately for each contrast.
Noise vs silence. Noise produces foci of activation in the region of HG and/or PT in all listeners. The activation appears along the posterolateral edge of HG for some listeners (2, 3, 4, 7) but not others (1, 5, 6, 8). There is a region of activation at the posteromedial end of HG for some listeners (1, 2, 4 and 5) but not others (3, 6, or 8), and there is a concentration of activation anterior to HG in some listeners (3, 5 and 7) but not others. In short, the noise activation in individuals is restricted to focal regions of HG and PT and together these regions produce the larger noise vs silence region in the group data. Within this larger region, however, the distribution of functional activation is quite variable across listeners. (The data of listener 9 are included in Figure 4 for completeness, but they are omitted from this analysis because the pattern of activation is so different from that of the other eight listeners.) This pattern of variation in the noise activation stands in marked contrast to the consistency of peaks across conditions within listeners; for any one of the small blue regions in an individual’s data, it is typically the case that the region is activated by all of the different sounds used in the experiment, and the peak in the region is often in exactly the same place in each condition. Moreover, the degree of activation is the same, inasmuch as these regions rarely appear in contrasts involving one of the pitch-producing sounds and noise, nor do they appear when these contrasts are inverted. The obvious hypothesis is that these regions of activation represent centres that analyse broadband sounds for specific features or properties other than pitch and they are in somewhat different places in different listeners.
Fixed vs noise
Centroids were calculated for the fixed vs noise peaks in each hemisphere for each listener and averaged across listeners; their co-ordinates are presented in the fourth row of Table 1. The functional centroid for the group is between the central and lateral anatomical centroids and a little below the line of anatomical centroids as in the group. The standard deviations for the functional centroids are a little greater than those for the anatomical centroids at the lateral end of HG but, in general, the variability of the functional centroids is comparable to that of the anatomical centroids for the fixed vs noise contrast. This relatively small, bilateral, region in lateral HG would appear to be a prime candidate for a pitch center or tone processing center in auditory cortex.
Melody vs fixed. There are three regions where melody produces more activation than fixed pitch, one at the lateral end of HG, one in STS, and one in PP. Figure 4 shows that there is somewhat more of this activity (green) in the individual data than in the group data (green and cyan in Figure 3), and the asymmetry is less pronounced in the individual data. There are two reasons for this: 1) the height threshold was reduced for the individual data (p < 0.001 uncorrected), and 2) the location of the activity is highly variable across listeners so much of it does not appear in the average. With regard to asymmetry, for 5 of the 9 listeners (1, 2, 3, 4, 6), there is more activation in the right hemisphere than the left; the activation is roughly balanced for listener 7, and listeners 5, 8 and 9 exhibit little or no melody vs fixed activity. In the right hemisphere, 6 of the 9 listeners have two regions of melody activation, but none has activity in all three of the regions identified in the average data, and no one combination of areas is overly common. In the left hemisphere, there is generally less activity and there is no discernible pattern. So, the distribution of activation associated with the processing of melody information outside auditory cortex is considerably more variable across listeners than the processing of pitch information in lateral HG. Melody produces essentially the same amount of activation as noise in PT and medial HG; in lateral HG, melody produces slightly more activation than fixed pitch, and so the regions of differential activation to melody (green) are largely separate from those associated with noise and fixed pitch. The exception is in lateral HG where listeners 2, 3, 6 and 7 exhibit some differential activation to melody within the region of activation associated with fixed pitch. It may be that lateral HG is involved in detecting when a pitch changes and the regions in PP and STS are involved in evaluating the pitch contour on a longer time scale.
Summary of listener variability. Eight of the nine listeners exhibit the hierarchy of melody processing observed in the group analysis. Within individuals, the locations of the peaks associated with the processing of noise and fixed pitch are highly consistent across stimulus conditions. Across listeners, however, the locations of the peaks associated with noise vary considerably. Similarly, the locations of peaks associated with the processing of melody in PP and STG vary considerably across individuals. The variability in the location of HG is small relative to the functional variability, and so the variability in the functional activation cannot be explained in terms of the gross anatomy.
DISCUSSION
With regard to the hierarchy of melody processing: The fact that the contrast between fixed pitch and noise activates a region lateral to PAC on HG, and the fact that the contrast between melody and fixed pitch produces activation outside of HG and PT, supports the hypothesis that there is a hierarchy of pitch processing in human cerebral cortex with the activation moving anterolaterally as processing proceeds. There are limited anatomical data concerning connectivity in human cerebral cortex, but the relevant studies (Howard et al., 2000; Tardif and Clarke, 2001; Hackett et al., 2001) are consistent with the hierarchy of processing suggested by the functional data. Anatomical studies of the macaque show that auditory cortex is composed of a central ‘core’, a surrounding ‘belt’ and a lateral ‘parabelt’ (Hackett et al., 1998) and that this cortical system is connected hierarchically (Kaas and Hackett, 2000). The core contains three regions: A1, a more rostral region, R, and an even more rostro-temporal region, RT (e.g., Kaas and Hackett, 2000). A recent comparative study of auditory cortex in macaques, chimpanzees and humans (Hackett et al., 2001) suggests that core in macaques corresponds to the central three quarters of HG in humans. In our functional data, the centroid of the activity associated with pitch on HG (shown red in Figure 3) is somewhat lateral and anterior to the region of HG traditionally associated with PAC. This suggests that the pitch region corresponds to the R or RT region of core. It should be noted, however, that the centroid of the pitch center in our study is below the central axis of HG, which may mean that it is not R or RT. The extra activation produced by melodies in STG and lateral PP is outside the core area; the region of activation in STG may correspond to a parabelt region in macaque, but the region in lateral PP seems rather anterior to be a parabelt region. In any event, the functional hierarchy of melody processing revealed in humans would appear to be consistent with the hierarchy of anatomical connections reported for macaques. With regard to the emergence of asymmetry in the hierarchy: In the study of subcortical activation (Griffiths et al., 2001), the fixed vs noise contrast revealed symmetric activation in the brainstem and slightly asymmetric activation in the auditory thalamus with greater activation on the right; together these findings led to the conclusion that subcortical processing of temporal regularity was largely symmetric. With regard to auditory cortex, the PET study of Griffiths et al. (1998) revealed bilateral activation that increased with the strength of the pitch produced by the RI sounds, although the activation was somewhat asymmetric within respect to HG. It was centered on HG in the right hemisphere and towards the lateral end of HG in the left hemisphere. The data from the current study indicate that all the broadband stimuli produce bilateral, and relatively symmetric, activity in auditory cortex (HG and PT) (the blue and red regions of Figure 3). [Griffiths et al. (2001) present a single axial section of the fixed vs noise contrast (Figure 3b) to show that the subcortical temporal processing continues in auditory cortex, as would be expected; the significance threshold in that Figure was less stringent (p<.001, uncorrected) than in Figure 3 of the current paper and so the region of activation appears larger.] In the current study, asymmetry is largely limited to the melody vs fixed contrast, and it emerges as the activation moves from HG on to PP and STG. This interpretation is consistent with a host of neuropsychological studies. One of the earlier studies (Samson & Zatorre, 1988) shows that patients with anterior temporal-lobe resections (sparing HG) are more impaired when detecting a single note-change in a pattern of three notes if the resections are on the right. A recent study (Johnsrude et al., 2000) indicates that patients with anterior temporal-lobe excisions that encroach upon the anterolateral extremity of HG in the right hemisphere, but not in the left, are more impaired when discriminating the direction of a melodic contour. Similarly, functional neuroimaging studies, which show relatively more activity in the right hemisphere in response to melodic sounds, support the hypothesis that the asymmetry is greater in regions anterior to auditory cortex (e.g., Zatorre et al., 1994; see Zatorre et al., 2002, for a review). In their PET study, Griffiths et al. (1998) performed an interaction analysis to find areas where activity increased as a function of pitch strength more for melodies than for fixed pitch and found two pairs of relatively lateral regions – a posterior pair near the intersection of PT and STG (-58 -42 -2 and 72 -40 6) and an anterior pair on PP (-54 10 -18 and 58 12 -26). The contrast between melody and fixed pitch in the current fMRI experiment is reasonably comparable and Figures 2J and 2K show activation in similar regions to those in the PET study; the peaks in the posterior regions are at -62 -28 2 and 66 -30 -2 and those in the anterior regions are at -56 6 -10 and 54 14 -16. The activation in the fMRI study is more asymmetric than in the PET study, perhaps because it had greater spatial resolution. There is also a recent study that relates musical ability, magnetoencephalographic (MEG) responses to modulated sinusoids, and grey matter volume in HG (Schneider et al. (2002). They found that the volume of grey matter in HG was significantly greater in a group of professional musicians than amateur and non-musicians, and the professionals had stronger MEG responses in medial HG. The data were symmetric for amateur musicians and non-musicians (consistent with our data), but there was a small, significant, asymmetry for professional musicians. So for professional musicians, the asymmetry associated with melody may emerge one stage earlier in the hierarchy of melody processing.
The hemispheric specialization hypothesis : In their review of asymmetry in response to speech and musical sounds, Zatorre et al. (2002) conclude that the processing of sounds with musical pitch results in relatively more activity in the right hemisphere, whereas the processing of sounds with critical timing information, like syllables with plosive consonants, results in relatively more activity in the left hemisphere. They then go on to propose that the auditory system has developed ‘… parallel and complementary systems – one in each hemisphere – specialised for rapid temporal processing (left) or for fine spectral processing (right) respectively’ (page 40), and they draw an analogy with the Uncertainty Principle as it applies to time and frequency constraints in the spectrogram (their Box 2). Note that, the discussion of the data is in terms of pitch while the HS hypothesis and the analogy are described in terms of spectral processing. The activation associated with the pitch and melody of RI sounds appears to be largely compatible with the pitch and melody activation discussed by Zatorre et al. (2002), as noted above. It seems somewhat difficult, however, to reconcile the processing of RI sounds with the part of the HS hypothesis that says that the right hemisphere is specialised for fine spectral processing. The HS hypothesis suggests that pitch is the result of fine spectral processing in auditory cortex, or to be more specific, that pitch arises from the detection of harmonically related peaks in the tonotopic representation of the Fourier magnitude spectrum of the sound as it occurs in, or near, auditory cortex (the concept is illustrated in Figures 1a and 1b of Griffiths et al., 1998). This cannot be the case for the RI sounds in this study, since there are no harmonically related peaks across the tonotopic dimension of the representation at any level in the auditory system (Figure1, panel G of the current paper). Moreover, the fine-grain timing information observed in physiological responses at the level of the brainstem is not observed in auditory cortex. This is what led us to suggest that the differential activation in lateral HG is associated with the calculation of precise values for pitch and pitch strength from the heights and widths of the peaks in a representation something like that shown in panel K of Figure 1. It may be that the same area calculates precise spectral pitch values when the tonotopic representation in auditory cortex exhibits peaks, and that the specialization is not so much one of ‘fine spectral processing in auditory cortex’ to extract an accurate pitch estimate, but rather one of ‘accurate monitoring of the pitch information flowing from auditory cortex’, in subsequent centers concerned with whether the sound has the kind of stable pitch exhibited by musical notes, and what the intervals are when the pitch jumps from one note to another. A modified version of the HS hypothesis in which the specialization involves fine pitch tracking rather than spectral processing would appear to be in good agreement with the data, but in this case, the analogy with the Uncertainty Principle would seem somewhat tenuous.
CONCLUSION
All of these broadband sounds in this study produced activation, bilaterally, in a number of centers in HG and PT independent of whether they produced a pitch or whether the pitch was changing over time. The fixed-pitch stimuli produced more activation than noise in lateral HG, bilaterally. The noise and fixed-pitch stimuli did not produce any substantial regions of activation outside HG and PT. When the pitch was varied to produce a melody, the sound produced additional, asymmetric, activation in STG and lateral PP with relatively more activity in the right hemisphere. While it is not possible at this time to be precise about the mechanics of auditory information processing at each stage, if we assume that there are three stages of melody information processing, as proposed in the introduction, and they occur in the order specified, then the broad mapping from stage of processing to brain region would appear to be as follows: (1) The extraction of time-interval information from the neural firing pattern in the auditory nerve, and the construction of time-interval histograms (e.g., the rows of Figures 1C and 1H), probably occurs in the brainstem and thalamus. (2) Determining the specific value of a pitch and its salience from the interval histograms probably occurs in lateral HG (e.g., by producing a summary histogram as in Figures 1E and 1K and locating the first peak). (3) Determining that the pitch changes in discrete steps and tracking the changes in a melody probably occurs beyond auditory cortex in STG and/or lateral PP. It would appear to be these latter processes associated with melody processing rather than pitch extraction per se that give rise to the asymmetries observed in neuropsychological and functional neuroimaging studies.
EXPERIMENTAL PROCEDUR
Subjects. Nine normal-hearing listeners volunteered as subjects after giving informed consent (six male, three female, mean age 34.3 ± 8.9 years). None of the listeners had any history of hearing disorders or neurological disorders.
Stimulus generation. The stimuli were sequences of noise bursts and regular-interval (RI) sounds. A RI sound is created by delaying a copy of a random noise and adding it back to the original. The perception has some of the hiss of the original random noise and also a weak pitch with a frequency at the inverse of the delay time. The strength of this pitch increases when the delay-and-add process is repeated (Yost et al., 1996). When the pitch is less than about 125 Hz and the stimuli are high-pass filtered at about 500 Hz, the RI sounds effectively excite all frequency channels in the same way as random noise (Patterson et al., 1996); compare the frequency profiles in Figures 1G and 1B. The perception of pitch in this case is based on extracting time-intervals rather then spectral peaks from the neural pattern produced by the RI sound in the auditory nerve; compare the frequency profile in Figure 1 (G) with the time-interval profile (K). Quantitative models of the pitch of RI sounds based on peaks in the time-interval profile have proven highly successful (e.g. Pressnitzer et al., 2001). There were five conditions in the experiment: four sound conditions and a silent baseline. The sounds were sequences of 32 notes played at the rate of four notes/s (8 s total duration). Each note was 200 ms in duration and there were 50 ms of silence between successive notes. The sounds were (i) random noise with no pitch (noise) and three RI sounds in which the pitch was (ii) fixed for a given 32-note sequence (fixed), (iii) varied to produce novel diatonic melodies (diatonic), or varied to produced random note melodies (random). The pitch range for the diatonic and random melodies was 50 to 110 Hz. The pitch in the ‘fixed-pitch’ sequences was varied randomly between sequences to cover the same range as the melodies over the course of the experiment. All of the sounds were band-pass filtered between 500 and 4000 Hz using fourth-order Butterworth filters and presented to both ears at 75 dB SPL through magnet-compatible, high-fidelity electrostatic headphones (Palmer et al., 1998).
fMRI protocol. Sparse temporal sampling was used to separate the scanner noise and the experimental sounds in time (Edmister et al., 1999; Hall et al., 1999). Blood-oxygenation-level-dependent (BOLD) contrast-image volumes were acquired every 12 s, using a 2.0-T MRI scanner (Siemens, VISION, Erlangen) with gradient-echo-planar imaging (TR/TE = 12000 ms /35 ms). A total of 48 axial slices were acquired covering the whole brain. Each condition was repeated 48 times in random order. A T1-weighted MPRAGE high-resolution (1 x 1 x 1.5 mm) structural image was also collected from each subject on the same MR system. Further details are presented in Griffiths et al. (2001).
Data processing and analysis. Structural and functional data were processed and analysed using SPM99 (http://www.fil.ion.ucl.ac.uk/spm). The BOLD time series was realigned to the first image of the series and then the structural image was co-registered to these images and resampled to 2 x 2 x 2 mm resolution. The realigned BOLD images were normalized to the standard SPM EPI template (ICBM 152, Brett et al., 2002) using affine and smoothly nonlinear spatial transformations: the same normalization parameters were applied to the structural images. The resulting images are in standardized, “Talairach-like” space. The ICBM-152 template creates images that are a few millimeters displaced from the Talairach brain (Talairach and Tournoux, 1988), particularly in the superior-inferior (z) dimension (Brett et al., 2002). This is the reason for the phrase “Talairach-like”. Finally, the functional data were smoothed with a Gaussian filter of 5 mm (full width at half maximum). Fixed-effects analyses were conducted on each listener’s data (288 scans), and across the whole group of subjects (with a total of 2592 scans) using the general linear model. The height threshold for group activation was t=5.00 (p<0.05 corrected for multiple comparisons across the whole volume). It was reduced somewhat for the individual data to t=5.00 (p < 0.001 uncorrected).
Evaluation of individual anatomy. In order to observe the relationship between functional activation and macroscopic anatomy, the putative location of primary auditory cortex was identified in each listener. All four of the authors labelled the first transverse temporal gyrus (HG) in both hemispheres of each listener, using a software labelling package (MRIcro: http://www.psychology.nottingham.ac.uk/staff/crl/mricro.html) and the anatomical criteria suggested by Penhune et al. (1996). Both white and grey matter were included in the labelled volumes. In some listeners HG is duplicated or partially duplicated (Penhune et al., 1996; Leonard et al., 1998). In this study, the duplication was ignored and the labelling was restricted to the part of HG anterior to any dividing sulcus (Rademacher et al., 1993; 2001). So our estimates of the extent of primary auditory cortex are a little more conservative than is traditional (Rademacher et al., 2001). The labelling of the four judges was combined to produce a labelled volume of HG for each listener by including all voxels that two or more judges had labelled as part of HG. The result is a three-dimensional HG map that is co-registered with the individual’s functional data. Finally, a mean HG volume was created for the group by averaging across the nine individual labelled volumes.
Comparison of anatomy with previous studies. The location of HG obtained in the current study was compared with that obtained in one morphological study (Penhune et al., 1996) and one cytoarchitectonic study (Morosan et al., 2001, Rademacher et al., 2001): in the former, HG was identified on the MR scans of 20 individuals; in the latter PAC was identified in brains of 10 individuals. Probability volumes of HG were obtained from Penhune, and centroids were calculated for the volumes in a probability-weighted fashion (Hall, et al., 2002). Rademacher et al. (2001) report centroids for a normalized version of their PAC data (p. 677). To ensure comparability, we obtained the raw data for nine cases from Rademacher and colleagues, and we applied the default, smoothly nonlinear, normalization of SPM99 to the data. Centroids were then calculated for these maps using the same probability-weighted function applied to the Penhune maps. All three sets of centroids are listed in Table 2. Note that the estimates based on HG and PAC data are almost identical. Overall, the positions for HG estimated from the current study are in good agreement the positions derived in previous studies. The group centroid in the current study is more anterior on the right than on the left (t (8df) = 3.69, p <0.01), consistent with previous reports. A comparison with other studies shows a displacement of 5-7.7 mm in the left and 3.2-5.9 mm in the right hemisphere. There was a significant difference (t = 2.71, p < .05) in left/right-displacement when compared with the centroids of HG, while this displacement was not significantly different from the estimates of the centre of PAC (see Table 2). One-group t-tests, testing for significant displacement (2-tailed) between left and right hemispheres separately for x, y, and z revealed that only the x co-ordinate in the left, and the z co-ordinate in the right were significantly different in location from all three other estimates. Our x co-ordinate in the left is, on average, 5.2 mm (individual averages range 4.1-7.1 mm) more lateral than in the other estimates. Our z co-ordinate in the right is 2.0 mm inferior to the other estimates (individual average range 1.4-2.6 mm).
ACKNOWLEGEMENT
The research was supported by the UK Medical Research Council (G9900369, G9901257).
REFERENCES
Brett, M, Johnsrude, I.S., Owen, A. M. (2002). The problem of functional localization in the human brain. Nature Reviews Neuroscience 3, 243 – 249.
Edmister WB, Talavage TM, Ledden PJ, Weisskoff RM. (1999). Improved auditory cortex imaging using clustered volume acquisitions. Hum. Brain Map. 7, 89-97.
Ehret, G., and Romand, R. (1997). The central auditory system (New York: Oxford University Press). Griffiths, T.D., Büchel, C., Frackowiak, R.S.J. and Patterson, R.D. (1998). Analysis of temporal structure in sound by the brain, Nature Neuroscience, 1, 422-427.
Griffiths, T.D., Uppenkamp, S., Johnsrude, I., Josephs, O., and Patterson, R.D. (2001). Encoding of the temporal regularity of sound in the human brainstem. Nature Neuroscience 4, 633-637.
Guimares, A.R., Melcher, J.R., Talavage, T.M., Baker, J.R., Ledden, P., Rosen, B.R., Kiang, N.Y.S., Fullerton, B.C., and Weisskoff, R.M. (1998). Imaging subcortical activity in humans. Hum. Brain. Map. 6, 33-41
Hackett, T.A., Stepniewska, and Kaas, J.H. (1998). Subdivisions of auditory cortex and ipsilateral cortical connections of the parabelt auditory cortex in macaque monkeys. J Comp. Neurol. 394, 475-495.
Hackett, T.A., Preuss, T. M. and Kaas, J. H. (2001). Architectonic identification of the core region in auditory cortex of macaques, chimpanzees, and humans. J. Comp. Neurol. 441, 197-222.
Hall, D.A., Haggard, M.P., Akeroyd, M.A., Palmer, A.R., Summerfield, A.Q., Elliott, M.R., Gurney, E.M., and Bowtell, R.W. (1999). "Sparse" temporal sampling in auditory fMRI. Hum. Brain Map. 7, 213-223.
Hall, D.A., Johnsrude, I.J., Haggard, M.P., Palmer, A.R., Akeroyd, M.A., Summerfield, A.Q. (2002). Spectral and temporal processing in human auditory cortex. Cereb. Cortex 12, 140-149.
Howard, M.A., Volkov, I.O., Mirsky, R., Garell, P.C., Noh, M.D., Granner, M., Damasio, H., Steinschneider, M., Reale, R.A., Hind, J.E., and Brugge, J.F. (2000). Auditory cortex on the human posterior superior temporal gyrus. J. Comp Neurol. 416, 79-92.
Johnsrude, I.S., Penhune, V.B., and Zatorre, R.J. (2000). Functional specificity in the right human auditory cortex for perceiving pitch direction. Brain 123, 155-163.
Kaas, J.H. and Hackett, T.A. (2000). Subdivisions of auditory cortex and processing streams in primates. Proc. Natl. Acad. Sci. 97, 11793-11799.
Krumbholz, K., Patterson, R.D., and Pressnitzer, D. (2000). The lower limit of pitch as determined by rate discrimination. J. Acoust. Soc. Am. 108, 1170-1180.
Leonard, C.M., Puranik, C., Kuldau, J.M., and Lombardino, L.J. (1998). Normal variation in the frequency and Location of human auditory cortex landmarks. Heschl's gyrus: where is it? Cereb. Cortex 8, 397-406.
Morosan, P., Rademacher, J., Schleicher, A., Amunts, K., Schormann, T, and Zilles, K. (2001). Human primary auditory cortex: subdivisions and mapping into a spatial reference system. Neuroimage 13, 684-701.
Palmer, A. R. and Winter, I. M. (1992). Cochlear nerve and cochlear nucleus responses to the fundamental frequency of voiced speech sounds and harmonic complex tones. In: Auditory physiology and perception. Y Cazals, L. Demany, K. Horner, eds. (Oxford: Pergamon), 231-239.
Palmer, A.R., Bullock, D.C., and Chambers, J.D. (1998). A high-ouput, high-quality sound system for use in auditory fMRI. Neuroimage 7, S359.
Patterson, R.D. (1994). The sound of a sinusoid: Time-interval models. J. Acoust. Soc. Am. 96, 1419-1428.
Patterson, R.D., Allerhand, M., and Giguere, C. (1995). Time-domain modelling of peripheral auditory processing: A modular architecture and a software platform. J. Acoust. Soc. Am. 98, 1890-1894.
Patterson, R.D., Handel, S., Yost, W.A., and Datta, A.J. (1996). The relative strength of the tone and noise components in iterated rippled noise, J. Acoust. Soc. Am. 100, 3286-3294.
Penhune, V.B., Zatorre, R.J., MacDonald, J.D., and Evans, A.C. (1996). Interhemispheric anatomical differences in human primary auditory cortex: probabilistic mapping and volume measurement from magnetic resonance scans. Cereb. Cortex 6, 661-672.
Pickles, J.O. (1988). An introduction to the physiology of hearing (2nd edition, London: Academic Press).
Pressnitzer, D., Patterson, R.D., and Krumbholz, K. (2001). The lower limit of melodic pitch. J. Acoust. Soc. Am. 109, 2074-2084.
Rademacher, J., Caviness, V.S., Steinmetz, H., and Galaburda, A.M. (1993). Topographical variation of the human primary cortices and its relevance to brain mapping and neuroimaging studies. Cereb. Cortex 3, 313-329.
Rademacher, J., Morosan, P., Schormann, T., Schleicher, A., Werner, C., Freund, H.J, and Zilles, K. (2001). Probabilistic mapping and volume measurement of human primary auditory cortex. Neuroimage 13, 669-683.
Rauschecker, J.P. and Tian, B. (2000). Mechanisms and streams for processing ‘what and ‘where’ in auditory cortex. Proc. Natl. Acad. Sci. 97, 11800-11806.
Rivier, F., and Clarke, S. (1997). Cytochrome oxidase, acetylcholinesterase, and NADPH-diaphorase staining in human supratemporal and insular cortex: evidence for multiple auditory areas. Neuroimage 6, 288-304.
Samson, S., Zatorre, R.J. (1988). Melodic and harmonic discrimination following unilateral cerebral excision. Brain Cogn 7, 348-60.
Talairach, P. and Tournoux, J. (1988). Co-planar stereotaxic atlas of the human brain. (Stuttgart: Thieme).
Tardif, E., and Clarke, S. (2001). Intrinsic connectivity in human auditory areas: tracing study with DiI. European Journal of Neuroscience 13, 1045-1050.
Wessinger, M., Van Meter, J., Tian, B., Pekar, J., and Rauschecker, J.P. (2001) Hierarchical organisation of the human auditory cortex revealed by functional magnetic resonance imaging. J. Cogn. Neurosc. 13, 1-7.
Winer, J. A. (1992). The functional architecture of the medial geniculate body and the primary auditory cortex. In: The mammalian auditory pathway: Neuroanatomy. D.B. Webster, A.N. Popper, and R.R. Fay, eds. (New York: Springer-Verlag), 222-409. Yost, W.A. (1996). Pitch of iterated rippled noise. J. Acoust. Soc. Am. 100, 511-518.
Yost, W.A., Patterson, R.D. and Sheft, S. (1996). A time-domain description for the pitch strength of iterated rippled noise, J. Acoust. Soc. Am. 99, 1066-1078.
Yost, W.A. (1998). Auditory processing of sounds with temporal regularity: Auditory processing of regular-interval stimuli. In: Psychophysical and physiological advances in hearing, A. Palmer, A. Rees, Q. Summerfield and R. Meddis, eds. (London: Whurr) 546-553.
Zatorre, R.J., Evans, A.C., and Meyer, E. (1994). Neural mechanisms underlying melodic perception and memory for pitch. J. Neurosci. 14, 1908-1919.
Zatorre, R.J., Belin, P., and Penhune, V.B. (2002). Structure and functions of auditory cortex: music and speech. Trends in Cog. Sci. 6, 37-46.