Sustained magnetic fields reveal separate sites for sound level and temporal regularity in human auditory cortex
From CNBH Acoustic Scale Wiki
Magnetoencephalography (MEG) was used to investigate the relationship between the sustained magnetic field in auditory cortex and the perception of periodic sounds. The response to regular and irregular click trains was measured at three sound intensities. Two separate sources were isolated adjacent to primary auditory cortex: One, located in lateral Heschl’s gyrus, was particularly sensitive to regularity and largely insensitive to sound level. The second, located just posterior to the first in planum temporale, was particularly sensitive to sound level and largely insensitive to regularity. This double dissociation to the same stimuli indicates that the two sources represent separate mechanisms; the first would appear to be involved with pitch perception and the second with loudness. The delay of the offset of the sustained field was found to increase with inter-click interval up to 200 ms at least, which suggests that the sustained field offset represents a sophisticated offset-monitoring mechanism rather than simply the cessation of stimulation.
Contents |
INTRODUCTION
Everyday sounds can be divided into three categories: transients like ticks and plops, noises like wind in the trees, and tones1 such as musical notes and vowels. Tonal sounds are produced by periodic or quasi-periodic waveforms and when the repetition rate of the waveform is greater than about 30 Hz, they produce static auditory perceptions with fixed pitch, loudness, and sound quality. The purpose of this paper was to determine the relationship between the magnetic field produced in auditory cortex in response to click trains and the regularity and level of the stimuli. The results revealed that two sources produce sustained fields in response to click trains, and the pattern of magnetic responses to changes in regularity and level is different for the two sources. As a result, they can be localised and identified with the type of information they process.
Auditory perception
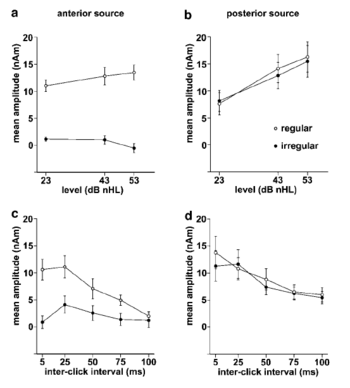
The auditory concepts of interest can be illustrated by considering the perceptions and physiological responses produced by regular and irregular click trains which, as the name implies, are just streams of acoustic impulses. Physiological recordings in the auditory nerve show that the individual clicks in these trains are separately represented at this level in the system (Young and Sachs, 1979). Computational models of auditory perception can now simulate the neural activity patterns (NAP) produced in response to a given sound (e.g., Patterson et al., 1995) and Fig. 1 shows the simulated response to a regular click train with an ICI of 5 ms (200 Hz repetition rate) at the level of the auditory nerve. The abscissa is time and the ordinate is place along the basilar membrane in the cochlea. This is the tonotopic dimension of hearing and it corresponds to frequency on a quasi-logarithmic scale. Each line in the figure represents the aggregate firing rate of all the fibers in a small region of the basilar membrane referred to as a frequency channel; for details see Patterson et al. (1995). The responses to each click is broader in time than the original click because of the filtering process applied in the cochlea, but in the frequency channels above about 2000 Hz, the click responses are still separate in time even at this relatively high click rate. Nevertheless, we do not hear individual clicks when presented with this sound; the clicks merge to form a tone which sounds something like a bassoon playing the G below middle C on the keyboard. This indicates that there must be a stage of temporal integration in the auditory system beyond the cochlea but before the level that corresponds to perception.
This is not in itself surprising, however, it has become clear that this temporal integration is not simple temporal averaging as in the visual system (Yost et al., 1998; Krumbholz et al., 2001). Patterson and coworkers have proposed a form of time-interval-sensitive temporal integration to explain recent findings. It is argued that, within each frequency channel (each line in Fig. 1), the system computes the time intervals between peaks in the neural activity pattern (NAP) and it constructs a time-interval histogram for that frequency channel. The resulting array of time-interval histograms is assumed to form the basis of the auditory image that we hear (Patterson et al., 1992, 1995) in response to a sound. The simulation produced by this Auditory Image Model (AIM) in response to the 200-Hz click train is presented in Fig. 1b; the abscissa is now time-interval rather than time. The details of the integration process are not crucial to the current study; the important points are 1) the neural pattern is fixed in position and stable in time so long as the sound continues unchanged, and 2) there is a concentration of activity in every channel at the time interval corresponding to the period of the sound, 5 ms, and at multiples of this time interval. Perceptual experiments have shown that the position of the first vertical ridge of activity in the auditory image (ignoring the one at 0 ms) predicts the pitch that listeners hear, and the height of the first ridge, relative to the overall level of activity, predicts the salience of the pitch (Yost et al., 1996; Patterson et al., 1996). With regard to MEG, the question is whether the magnetic response represents the detection of a stable structure produced by a regular sound in the auditory image (and/or its pitch). If so then the magnetic response should appear in response to regular click trains and not in response to irregular click trains. Moreover, the response should not vary markedly with sound level because the pitch depends on the position of the first vertical ridge rather than its level, and the salience is measured relative to the total activity in the image and so it should not vary with stimulus intensity either. Irregular click trains with average rates in the range 10 to 200 clicks per second, sound like a Geiger counter at varying distances from a radioactive source; the perceptions have no pitch. The NAP and auditory image produced in response to an irregular click train with an average ICI of 5 ms are presented in Figs 1b and 1d, respectively. The NAP shows that the clicks produce individual responses that are separated in time in the channels above about 2000 Hz as in the case of the regular click train. However, the time intervals in the NAP of Fig. 1b are not regular and so there are no concentrations of activity at particular time intervals in the auditory image of the irregular click train (Fig. 1d). The irregular click train has the same energy as the regular click train and it has essentially the same loudness. In AIM, the loudness of a click train corresponds to the sum of all of the activity in the auditory image. On average, there are the same number of clicks in the regular and irregular click trains and on average there are the same number of time intervals in the NAP, and so the sum of the activity in the auditory image is essentially the same for the two types of click train. The difference is just that the activity for the regular click train is concentrated on the structure in the image. With regard to MEG, the question is whether the magnetic response represents the loudness of the sound. If so then it should appear in response to both regular and irregular click trains and the responses should have about the same magnitude. More interestingly, if there are separate centres in auditory cortex for the analysis of pitch and stability, on the one hand, and loudness on the other, and if these centres are sufficiently separated spatially in auditory cortex, then the magnetic response might enable us to isolate and identify both of them.
MEG measures of auditory activity
Magnetoencephalography (MEG) combines the high temporal resolution required to study temporal processing with sufficient spatial resolution to localize neocortical generators. There are two responses that have been used to investigate the processing of periodic sounds in auditory cortex. One is the 40-Hz steady-state response (Mäkelä and Hari, 1987; Gutschalk et al. 1999) associated with time-locked activity in primary auditory cortex (AI). The other is the sustained response (Köhler et al., 1952; Hari et al., 1980) that persists for the duration of sustained sounds more than about 100 ms in duration (Picton et al., 1978a). The sustained response builds up over 50–200 ms (Picton et al., 1978a, Scherg et al., 1989) and returns to baseline shortly after stimulus cessation. There are transient peaks in the response at 100 and 200 ms, referred to as N100 and P200, which can overlap with both the onset and offset of the sustained response (Hillyard and Picton, 1978; Scherg et al., 1989; Pantev et al., 1996). The transient N100 and P200 have been separated from the sustained response in parametric studies involving tonal sounds (Picton et al., 1978a) and speech sounds (Euliz et al. 1995). They can also be segregated with source analysis techniques (Hari et al., 1985; Scherg et al., 1989; Pantev et al. 1994, 1996; Eulitz et al., 1995). Regular click trains with rates in the range of 40 Hz produce a strong sustained field in humans (Mäkelä and Hari 1987; Gutschalk et al., 1998). Epicortical recordings in cats indicate that the response becomes sustained as click rate rises from 5–19 Hz (Gumnit 1960). This might suggest that the sustained field is associated with the processing of periodic sounds. It is also the case, however, that the amplitude of the sustained potential is correlated with stimulus intensity and inter-stimulus interval (David et al., 1969; Picton et al., 1978b) which might suggest that the sustained field simply reflects the sum of all neural activity in auditory cortex, that is, the loudness of the sound. In any event, the sustained field is clearly associated with the processing of continuous sounds in auditory cortex and so it is this MEG measure that is the focus of this paper.
MATERIAL AND METHODS
The relationship between the sustained field and the properties of click trains was investigated in two experiments: In the first, the response to regular and irregular click trains was measured as a function of average click rate from 200 to 10 Hz. In the second, the level of the click trains was varied from 23 to 53 dB nHL, again with both regular and irregular click trains.
Stimuli
The stimuli consisted of one set of regular and one set of irregular click trains, each 1 s in duration. The ICIs in the regular condition were 5, 25, 50, 75, or 100 ms. In the irregular condition, the ICIs were chosen randomly and uniformly from the interval ICI±½ICI, so the ICI range for the 5-ms condition, for example, was 2.5–7.5 ms. Click trains were presented in pseudo-randomized order at an inter-train interval of 0.8–1.2 s. Each ICI condition was repeated 180 times and all of the trials within the condition were presented in one block; in the irregular condition there was a different temporal structure for each repetition of a stimulus. In the first experiment, absolute threshold was measured for the regular, 5-ms click trains to establish normal hearing level (nHL), and the sound level of these click trains was set to 43 dB nHL. The intensity of the individual clicks was kept constant for all of the other conditions. Stimuli were presented binaurally using shielded transducers (Neuroscan Inc.) with 90-cm plastic tubes and foam ear pieces. The digital waveform of a single click was rectangular shaped at 31.25 s duration (one sample up at 32 kHz sampling rate). The transceivers act as bandpass filters whose effective bandwidth is from approximately 500 to 3000 Hz. In the second experiment, 5-ms regular and irregular click trains were presented at sound levels of 23, 43 and 53 dB nHL. In this experiment, both regular and irregular stimuli were presented within one large block, to allow for an analysis based on the subtraction of conditions. Otherwise, the procedure was very similar to that of the first experiment.
Subjects
Twelve right-handed subjects (6 male and 6 female) with no history of peripheral or central hearing disorder participated in each experiment after giving informed consent. The mean age was 28 years in the first experiment (range 24–37 years) and 30 years in the second experiment (range 24–37 years). Nine of the subjects participated in both studies. During recording, subjects watched a self-selected silent movie. They were instructed not to pay attention to the experimental sounds.
Recording and Data Processing
Data were acquired with a Neuromag-122TM whole-head MEG system inside a magnetically shielded room. Recordings were made with a 1000-Hz sampling rate through a lowpass filter with a cutoff frequency of 330 Hz and no highpass filter (direct coupling). Horizontal and vertical electrooculograms were recorded simultaneously to control for ocular artifacts. Data analysis was performed with the BESA®2000 software package (MEGIS Software GmbH). Auditory evoked fields were averaged off-line over an epoch from 400 ms before to 1600 ms after click-train onset. Prior to averaging, the data were inspected to exclude slow external artifacts. Abrupt changes in amplitude with gradients of more than 600–800 fT per sample were discarded automatically. Altogether, about 10 % of the sweeps were rejected by one method or the other. A baseline amplitude, calculated over the 200-ms interval before the onset of the click train was subtracted from the data.
Source Analzsis
Initially, the data were analysed with a conventional MEG model (Hari et al., 1987; Scherg et al., 1989; Pantev et al., 1994, 1996) comprising one dipole in each auditory cortex to estimate the equivalent dipole sources of the sustained field in the two hemispheres (Scherg and von Cramon, 1985; Scherg, 1990). This two-dipole model was adapted for each individual, with the regular, 5-ms ICI click train condition as it provided the best signal-to-noise ratio; the data were lowpass filtered at 60 Hz (zero-phase shift, butterworth filter, 24 dB/oct). A non-constrained, bilateral pair of dipoles was fitted to the epoch 500–950 ms post stimulus onset where the sustained field was stable and appeared to be free from the influence of onset and offset generators. A spherical head model was used with the center positioned 5 mm anterior and superior to the posterior commissure. In the second experiment, interleaved presentation of the regular and irregular click trains enabled us to test the hypothesis that there is a second source for regular stimuli. The procedure is based on the subtraction of regular and irregular conditions. A model with two sources in each hemisphere of auditory cortex was created, one reflecting the neuronal activation in auditory cortex that is similar for both, regular and irregular conditions, and a second one that depicts the additional activation induced by regularity. To achieve sufficient signal-to-noise ratio, this four-dipole model was fitted using the data from all three sound levels. To increase the signal to noise ratio further the data were lowpass filtered at 20 Hz (zero-phase shift, butterworth filter, 24 dB/oct.). Two dipoles were fitted to the irregular condition to model the activity contained in both conditions. Similarly, two dipoles were fitted to the difference condition, regular minus irregular, to model the additional activity in the response to regularity. All four dipoles where then combined into a multi-dipole model for use as a spatial filter to derive source waveforms for all conditions. To compensate for the slow magnetic artifacts inherent in DC recordings, we estimated the artifact by computing a principle component analysis (PCA) for each average condition over the epoch 450–550 ms post stimulus offset. . The PCA component explaining the majority of variance in this epoch was the slow magnetic drift and it was included in the spatial filter for each individual condition (Berg and Scherg, 1994). The same procedure was applied to the two-dipole-model analysis. For statistical comparison of sustained field amplitudes, we calculated mean amplitude values over the epoch 500–1000 ms post train onset for each source. Analysis of variance was performed with SAS (version 6.12), using the general linear model for repeated measurement. The offset latency of the sustained field was calculated by searching for the minimum in the first derivative of the source waveforms, in the range from 50–350 ms post stimulus offset (3-Hz low pass filter, 24 dB/oct). This inflection point provides a robust estimate of the latency where the sustained field is half way back to baseline. The dipole locations were mapped onto individual T1-weighted MRI scans and transformed into the standard stereotactic space of Talairach and Tournoux (1988) using the BrainVoyagerTM software (Brain Innovation B.V.). To determine whether the source locations were independent, the transformed coordinates of the sources were tested for spatial separation in X, Y and Z directions using the general linear model for repeated measurements
RESULTS
Source Analzsis
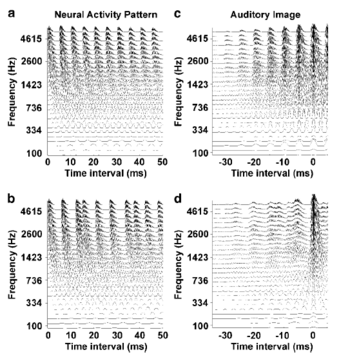
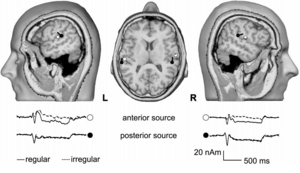
The initial two-dipole model produced a consistent fit with one dipole located in the central aspect of the first Heschl’s gyrus (Penhune et al., 1996; Leonard et al., 1998) in each hemisphere. The average Talairach coordinates (Talairach and Tournoux, 1988) are presented in Table 1. A similar two-dipole model was fitted to the level data and it produced very similar estimates of the source locations (Table 1). The dipole model (Scherg, 1990) for the first experiment was then used as a spatial filter to obtain source waveforms for the 12 subjects individually. The grand average source waveforms for each ICI are shown in Fig. 2a.
Repetition Rate and Regularity
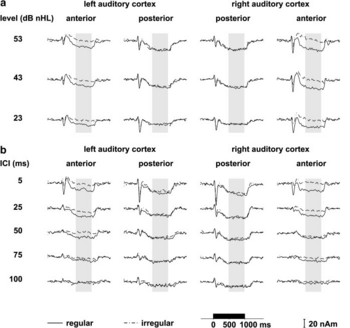
Over the epoch from 500–1000 ms, the sustained field was effectively constant in all ICI conditions. Within this interval, the average amplitude of the sustained field to regular click trains decreased monotonically with increasing ICI (Fig. 2b). The sustained fields recorded to irregular click trains were significantly smaller in both hemispheres at all repetition rates (F1,11=22.46, p<0.001). This difference was evident in all subjects but one, whose sustained field to regular trains was larger in the right hemisphere, while largely unchanged in the left hemisphere. The difference was largest for the shortest ICI, 5 ms, and there was a highly significant interaction between ICI and regularity (F4,44=17.08, p<0.0001). At the onset, the sustained field is accompanied by a large tri-phasic transient wave which precluded analysis of the onset properties of the sustained component. In contrast, the offset was not accompanied by any obvious transient peaks, and we were able to calculate the offset-latency of the sustained field. The lack of a transient off-response might be due to the relatively short duration of stimuli (Hillyard and Picton, 1978) on the one hand, or to the stimulation with click trains on the other hand. Support for this view comes from two previous studies using click trains (Mäkelä and Hari 1987, Gutschalk et al. 1998). They also found no transient off-response with click trains, while with noise bursts, a transient peak was often recorded at the offset when the stimuli were 400 ms in length (Hari et al. 1987). The data showed that the offset-latency increased almost linearly with ICI (F4,44=189.29, p<0.0001) from 108 to 256 ms as the ICI increased from 5 to 100 ms (Fig. 2). To investigate whether this offset delay persists at intervals longer than 100 ms, we presented 1-second click trains with ICIs from 100 to 500 ms to six subjects. They all showed a reliable sustained field for an ICI of 200 ms; at longer ICIs the pattern became inconsistent. The offset latency increased montonically up to about 350 ms for the 200-ms ICI.
Intensity and Regularity
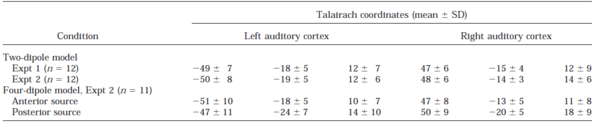
The interaction of regularity and intensity was studied with 5-ms, regular and irregular trains at levels of 23, 43, and 53 dB nHL. Fitting two dipoles to the irregular data and two to the difference conditions, regular minus irregular, enabled separation of two different processes (Fig. 3). The strategy yielded a stable model for 11 of the 12 subjects; the complete four-dipole model could not be established for the subject mentioned above who showed a similar response to regular and irregular stimuli in the left hemisphere. Accordingly, this subject was excluded from further analysis. The average Talairach coordinates (Table 1) indicate that the source associated with the difference condition is located in the central aspect of the first Heschl’s gyrus (Penhune et al., 1996; Leonard et al., 1998). The source associated with the irregular condition is more posterior, on the border between Heschl’s gyrus and the planum temporale (Leonard et al., 1998). The locations of the two sources were significantly different (F2,20=10.87, p<0.001) with the majority of the variance explained in the anterior-posterior direction. The grand-average source waveforms corresponding to the four dipolar sources are plotted in Fig. 4a. Fig. 5 (a & b) presents the amplitudes of the two sources as a function of sound level. As there was no significant difference between hemispheres the amplitudes were averaged across hemispheres. The anterior source exhibited markedly stronger sustained fields for regular click trains (Fig. 5a; F1,10=132.34, p<0.0001). There was no significant main effect of sound level (F2,20=0.34, n.s.) but there was an interaction of regularity with level (F2,20=4.26, p<0.05), indicating that the anterior sustained field might not be entirely independent of level. In contrast, the posterior source exhibited a strong effect of level (Fig. 5b; F2,20=20.17, p<0.0001) and no difference between regular and irregular conditions (Fig. 5b; regularity: F1,10=0.97, n.s.; regularity*intensity: F2,20=1.54, n.s.).
Effect of Repetition Rate in the Four-Dipole Model
It was not possible to fit a four-dipole model to the data of the ICI experiment because the regular and irregular conditions were performed separately. For eight subjects who participated in both experiments, however, it was possible to use the four-dipole model fitted to the level data as a spatial filter to derive source waveforms from the ICI data (Fig 4b). The spatial filter was created by adjusting the dipole model to the individual head position for that recording session; for each individual, the estimate of the slow magnetic drift was included for each condition, separately, as described in the methods section. The amplitude of the anterior source (Fig. 5c) was stronger for regular stimuli (F1,7=23.44, p<0.01) and more so at shorter ICIs (ICI*regularity: F4,28=7.25, p<0.001). As ICI increased, amplitude decreased for both regular and irregular stimuli (F4,28=7.49, p<0.001) and at 100 ms, it was close to baseline. The amplitude of the posterior source also decreased as ICI increased (Fig. 5d) from a mean of about 13 nAm at 5 ms to 6 nAm at 100 ms (F4,28=9.22, p<0.0001); in contrast to the anterior source, there was no significant difference between regular and irregular conditions (F1,7=0.85, n.s.). Since the amplitude in the anterior source was very small at long ICIs, the offset latency could not be reliably estimated; to the extent that they were measurable, however, there was no significant difference between anterior and posterior sources. The dominance of the posterior source at long ICIs suggests that the increase of the offset latency for ICIs longer than 50 ms is produced solely by the posterior generator, while at shorter ICIs both processes may contribute to the offset delay.
DISCUSSION
The MEG data reveal that there are two sources that produce a sustained field, one of which is highly sensitive to temporal regularity and insensitive to level, and one of which is insensitive to regularity and highly sensitive to level. In this section we discuss the implications of this double dissociation, along with previous findings on auditory processing and the anatomy of auditory cortex.
The Anterior Sustained Field
The effect of temporal regularity on the anterior source is most pronounced at the shorter ICIs (5 and 25 ms) where the stimuli produce a clear pitch. The fact that the effect exists for ICIs as long as 25 ms means that it is not dependent on the frequency resolution provided by cochlear filtering since the Fourier components of a click train with an ICI of 25 ms (40 Hz) would not be resolved by the cochlea. Moreover, tests with one listener showed that highpass filtering at the tenth harmonic of the repetition rate did not eliminate the effect of regularity, although it would certainly have removed any resolved harmonics. The fact that the sustained field fades away as ICI increases beyond 25 ms supports the hypothesis that this generator is concerned with pitch processing; perceptual data show that pitch fades away as ICI increases over this same range (25 - 100 ms) (Krumbholz et al., 2000; Pressnitzer et al., 2001). Using positron emission tomography, Griffiths et al. (1998) showed that time-interval processing like that involved in analyzing the regularity of click trains has occurred by the time the response to the stimulus has reached the level of secondary auditory cortex. Our data suggest that the neuronal activity that produces the blood flow changes they observed, also produces the sustained field associated with our anterior source. While our data cannot answer the question of where the auditory system decodes stimulus regularity, it adds evidence to their hypothesis that the process is completed by the time the response reaches secondary auditory cortex. Animal studies provide evidence for the transformation of the time-locked code observed in the inferior colliculus into a rate-place code at the level of the auditory cortex (Langner and Schreiner, 1988; Schulze and Langner, 1997). Recent studies have, however, shown that arrival time information exists in primary auditory cortex for click rates up to 300 Hz (Steinschneider et al., 1998; Rupp et al., 2000), indicating that the temporal integration that produces stability does not completely remove transient information from the representation at the level of primary auditory cortex. The fact that the anterior sustained field is greater for regular click trains and largely independent of level suggests that the generator is operating on some aspect of regularity or staability in the perception, like the fixed position of the structure produced by the periodic click trains in the auditory image (Patterson et al., 1992). In the model, pitch is derived from the peak at the time interval associated with period, which fades away like the sustained field as ICI increases beyond 25 ms.
The Posterior Sustained Field
The posterior sustained field is largely insensitive to the presence or absence of regularity in click trains and it is sensitive to level which suggests that it is concerned with the loudness of the sound rather than the pitch. At the longer ICIs, the posterior generator dominates the overall amplitude of the sustained field and it is probably the only contributor for ICIs greater than 100 ms. Reliable sustained fields were recorded for an ICI of 200 ms, which corresponds to a lower click rate than that found to elicit a sustained potential in cat auditory cortex (Gumnit, 1960). These amplitude data might suggest that the posterior source is an exponential leaky integrator which averages the activity evoked by the individual clicks over 200–300 ms (Zwislocki, 1969). This would explain the effects of rate and intensity on the amplitude of the posterior sustained field.
The Offset Delay Effect
The leaky integrator interpretation is contradicted, however, by the offset latency data which suggest that the offset mechanism is more sophisticated. It is the ICI rather than the amplitude of the sustained field that appears to determine the offset delay. The amplitude prior to offset is greater for shorter ICIs. If the temporal integration properties of the mechanism were like those of a leaky integrator, the larger amplitudes associated with shorter ICIs would lead to offset decay functions that were greater than those for longer ICIs at all post-stimulus times. The reverse is observed: longer ICIs, associated with lower amplitudes, lead to longer offset delays. Moreover, the shape of the offset function is not exponential; it is more like an ojive (cumulative Gaussian) and there was little evidence of a transient off-response (Hillyard and Picton, 1978; Pantev et al., 1996). Together these observations suggest that the mechanism is more concerned with flagging the existence of an auditory event and the time of its offset. It is as if the posterior generator is involved with characterizing the overall level of activity of a sound source, independent of regularity, and specifying when the source of the activity terminates. For click trains, it has to wait after each click for at least the duration of the average ICI to see if another click will occur before it can specify that an offset has occurred. For click trains with longer ICIs it has to wait longer even though they produce a smaller sustained field. The generator may also be involved in specifying the onset of a sound source but that part of the waveform is obscured by the onset response in these experiments. A similar mechanism may apply to the anterior sustained field as well, binding together single components of a sound to an object of distinct pitch and duration.
Anatomical Location of the Sustained Field Generators
The sustained field observed in auditory cortex in response to periodic sounds has been shown to have two components: an anterior source in Heschl’s gyrus and a posterior source at the border with planum temporale. Cytoarchitechtonic studies of human auditory cortex suggest that both generators are located near the border between konio- and para-koniokortex (Braak, 1978; Galaburda and Sanides, 1980; Rivier and Clarke, 1997), also referred to as the core and belt areas (Braak, 1978).
The location of the posterior source at the caudal border of the core, within 10-20% probability, suggests that it is generated in the adjacent posterior belt (Braak 1978, Galaburda and Sanides 1981). It should be noted, however, that the dipole analysis technique provides only the centre of activation; there is no information about the spatial extent of the sources. Therefore, we cannot determine whether the posterior source is generated exclusively in the posterior belt or if AI contributes to the posterior source as well. Epicortical recordings in cats (Gumnit, 1961) indicate that primary auditory cortex contributes to the sustained potential; however, the present results and a previous study (Gutschalk et al., 1998) indicate that it is unlikely that the koniofield is the only generator. Our current findings suggest that the lateral auditory core/belt and the posterior belt are specialized to process basic acoustic features of sound. In this specific case, the features are level and temporal regularity which are, of course, closely related to the basic perceptions loudness and pitch.
ACKNOWLEDGEMENTS
Research supported by the DFG (ZIZAS, grant SCHE-558/2-2) and the UK MRC (G9703469, G990369). The MRI data were provided by the Department of Neuroradiology, University of Heidelberg, Germany.
NOTES
1 In this paper, the word “tone“ is used as in common language to mean “A sound of definite pitch and character produced by regular vibration of a sounding body; a musical note“ (Oxford English Dictionary). The word is used to contrast the perceptions produced by periodic sounds (and quasi-periodic sounds like vowels and musical notes) with those produced by non-periodic sounds like noises and clicks. The word “tone“ should not be confused with the word “sinusoid“ which is a very specific form of periodic sound used in acoustics and psychoacoustics because of its unique energy distribution. Sinusoids and regular click trains are both classes of sound that produce tonal perceptions, but it is the latter that serves as the periodic (regular) sound in this paper.
REFERENCES
Berg, P., and Scherg, M. 1994. A multiple source approach to the correction of eye artifacts. Electroenceph. clin. Neurophysiol. 90:229-241.
Braak, H. The pigment architecture of the human temporal lobe. 1978. Anat. Embryol. 152:141-169. David, E., Finkenzeller, S., Kallert, S., and Keidel, W.D. 1969. Akustischen Reizen zugeordnete Gleichspannungsänderungen am intakten Schädel des Menschen. Pflügers Arch. 309:362-367.
Eulitz, C., Dietsch, E., Pantev, C., Hampson, S., and Elbert, T. 1995. Magnetic and electric brain activity evoked by the processing of tone and vowel stimuli. J. Neurosci. 15:2748-2755.
Flanagan, J.L., and Guttman, N. 1960. On the pitch of periodic pulses. J. Acoust. Soc. Am. 32:1308-1319.
Galaburda, A., and Sanides, F. 1980. Cytoarchitectonic organization of the human auditory cortex. J. Comp. Neurol. 190:597-610.
Griffiths, T.D., Büchel, C., Frackowiak, R.S.J., and Patterson, R.D. 1998. Analysis of temporal structure in sound by the brain. Nat. Neurosci. 1:422-427.
Gumnit, R.J. 1960. D.C. potential changes from auditory cortex of the cat. J. Neurophysiol. 23:667-675.
Gumnit, R.J. 1961. The distribution of direct current responses evoked by sounds in the auditory cortex of the cat. Electroenceph. clin. Neurophysiol. 13:889-895.
Gutschalk, A. Scherg, M., Picton, T.W., Mase, R., Roth, R., Ille, N., Klenk, A., and Hähnel, S. 1998. Multiple source components of middle and late latency auditory evoked fields. In Recent Advances in Human Neurophysiology (I. Hashimoto, and R. Kakigi), pp 270-278. Elsevier Science, Amsterdam.
Gutschalk, A., Mase, R., Roth, R., Ille, N., Rupp, A., Hähnel, S., Picton, T.W., and Scherg, M. 1999. Deconvolution of 40 Hz steady-state fields reveals two overlapping source activities of the human auditory cortex. Clin. Neurophysiol. 110:856-868.
Hackett, T.A., Stepniewska, I., and Kaas, J.H. 1998. Subdivisions of auditory cortex and ipsilateral cortical connections of the parabelt auditory cortex in macaque monkeys. J. comp. Neurol. 394: 475-495.
Hari R., Aittoniemi K., Järvinen M.L., Katila T., and Varpula T. 1980. Auditory evoked transient and sustained magnetic fields of the human brain. Exp. Brain Res. 40:237-240.
Hari, R., Pelizzone, M., Mäkelä, J.P., Hällström, J., Leinonen, L., and Lounasmaa, O.V. 1987. Neuromagnetic response of the human auditory cortex to on- and offset of noise bursts. Audiology 26:31-43.
Hillyard, S.A., and Picton, T.W. 1978. On and Off components in the auditory evoked potentials. Perception & Psychophysics 24:391-398.
Köhler, W., Held, R., and O’Connell, D. 1952. An investigation of cortical currents. Proc. Amer. Philos. Soc. 96:290-330.
Krumbholz, K., Patterson, R.D., and Pressnitzer, D. 2000. The lower limit of pitch as determined by rate discrimination. J. Acoust. Soc. Am. 108:1170-1180.
Krumbholz, K., Patterson, R. D., and Nobbe, A. 2001. Asymmetry of masking between noise and iterated rippled noise: Evidence for time-interval processing in the auditory system. J. Acoust. Soc. Am. 110:(in press, Sept/Oct).
Langner, G., and Schreiner, C.E. 1988. Periodicity coding in the inferior colliculus of the cat: I. Neuronal mechanisms. J. Neurophysiol. 60:1799-1822.
Leonard, C.M., Puranik, C., Kuldau, J.M., and Lombardino, L.J. 1998. Normal variation in the frequency and location of human auditory cortex landmarks. Heschl’s Gyrus: Where is it? Cereb. Cortex 8:397-406.
Mäkelä, J.P., and Hari, R. 1987. Evidence for cortical origin of the 40 Hz auditory evoked response in man. Electroenceph. clin. Neurophysiol. 66:539-546.
Morel, A., Garraghty, P.E., and Kaas, J.H. 1993. Tonotopic organization, architectonic fields, and connections of auditory cortex in macaque monkeys. J. Comp. Neurol. 273: 52-66.
Morosan, P., Rademacher, J., Schleicher, A., Amunts, K., Schormann, T., and Zilles, K. 2001. Human primary auditory cortex: Cytoarchitectonic subdivisions and mapping into a spatial reference system. NeuroImage 13: 684-701.
Pantev, C., Eulitz, C., Elbert, T., and Hoke, M. 1994. The auditory evoked sustained field: origin and frequency dependence. Electroenceph. clin. Neurophysiol. 90:82-90.
Pantev, C., Eulitz, C., Hampson, S., Ross, B., and Roberts, L.E. 1996. The auditory evoked “off“ response: Sources and comparison with the “on“ and the “sustained“ response. Ear Hear. 17:255-265.
Patterson, R.D. (1994) The sound of a sinusoid: Time-interval models. J. Acoust. Soc. Am. 96:1419-1428.
Patterson, R.D., Allerhand, M., and Giguere, C. 1995. Time-domain modelling of peripheral auditory processing: A modular architecture and a software platform. J. Acoust. Soc. Am. 98:1890-1894.
Patterson, R.D., Handel, S., Yost, W.A., Datta, A.J. 1996. The relative strength of the tone and noise components in iterated rippled noise. J. Acoust. Soc. Am. 100: 3286-3294.
Patterson, R.D., Robinson, K., Holdsworth, J.W., McKeown, D., Zhang, C., and Allerhand, M. 1992. Complex sounds and auditory images. In Auditory physiology and perception (Y. Cazals, L. Demany, and K. Horner), pp 429-446. Pergamon, Oxford.
Penhune, V.B., Zatorre, R.J., Mac Donald, J.D., and Evans, A.C. 1996. Interhemispheric anatomical differences in human primary auditory cortex: Probabilistic mapping and volume measurement from magnetic resonance scans. Cereb. Cortex 6:661-672.
Picton, T.W., Woods, D.L., and Proulx, G.B. 1978a. Human auditory sustained potentials. I. The nature of the response. Electroenceph. clin. Neurophysiol. 45:186-197.
Picton, T.W., Woods, D.L., and Proulx, G.B. 1978b. Human auditory sustained potentials. II. Stimulus relationships. Electroenceph. clin. Neurophysiol. 45:198-210.
Pressnitzer, D., Patterson, R. D., and Krumbholz, K. 2001. The lower limit of melodic pitch. J. Acoust. Soc. Am. 109, 2074-2084. Rademacher, J., Morosan, P., Schormann, T., Schleicher, A., Werner, C., Freund, H.-J., and Zilles, K. 2001. Probabilisitc mapping and volume measurement of human primary auditory cortex. NeuroImage 13: 669-683.
Rivier, F., and Clarke, S. 1997. Cytochrome oxidase, acetylcholinesterase, and NADPH-diaphorase staining in human supratemporal and insular cortex: Evidence for multiple auditory areas. NeuroImage 6:288-309.
Rupp, A., Hack, S., Gutschalk, A., Schneider, P., Picton, T.W., Stippich, C., and Scherg, M. 2000. Fast temporal interactions in human auditory cortex. Neuro Report 11:3731-3736.
Scherg, M., and von Cramon, D. 1985. Two bilateral sources of the late AEP as identified by a spatio-temporal dipole model. Electroenceph. clin. Neurophysiol. 62:32-44.
Scherg, M. 1990. Fundamentals of dipole source analysis. In Auditory Evoked Magnetic Fields and Electric Potentials, Advances in Audiology, vol. 6 (F. Grandori, M. Hoke, and G.L. Romani), pp 40-69. Karger, Basel.
Scherg, M., Vajsar, J., and Picton, T.W. 1989. A source analysis of the human auditory evoked potentials. J. Cogn. Neurosci. 1:336-354.
Schulze, H., and Langner, G. 1997. Periodicity coding in the primary auditory cortex of the mongolian gerbil (meriones unguiculatus): two different coding strategies for pitch and rhythm? J. comp. Physiol. A 181:651-663.
Steinschneider, M., Reser, D.H., Fishman, Y.I., Schroeder, C.E., and Arezzo, J.C. 1998. Click train encoding in primary auditory cortex of the awake monkey: Evidence for two mechanisms subserving pitch perception. J. Acoust. Soc. Am. 104:2935-2955.
Talairach, P., and Tournoux, J. 1988. A stereotactic coplanar atlas of the human brain, Thieme, Stuttgart.
Tsuzaki, M., and Patterson, R.D. 1998. Jitter Detection: A brief review and some new experiments. In Psychophysical and physiological advances in hearing. (A. Palmer, A. Rees, Q. Summerfield, and R. Meddis), pp 546-553. Whurr, London.
Yost, W.A., Patterson, R.D, and Sheft, S. (1996). "A time domain description for the pitch strength of iterated rippled noise," J. Acoust. Soc. Am. 99, 1066-1078.
Yost, W.A., Patterson, R.D. and Sheft, S. (1998). "The role of the envelope in processing iterated rippled noise," J. Acoust. Soc. Am. 104, 2349-2361.
Young, E.D., and Sachs, M.B. 1979. Representation of steady-state vowels in the temporal aspects of the discharge patterns of populations of auditory-nerve fibers. J. Acoust. Soc. Am. 66:1381-1403.
Zwislocki, J. 1969. Temporal summation of loudness: An Analysis J. Acoust. Soc. Am. 46:431-440.